Researchers at NYU Langone Health and its Center for Advanced Imaging Innovation and Research have created an AI model that can advise technologists conducting prostate MRI exams on whether to conclude the exam at an “abbreviated” stage or proceed to a more detailed protocol based on a real-time estimation of the patient’s cancer risk. Reported in the September issue of the Journal of Magnetic Resonance Imaging, the advance has the potential to personalize the current standard of care in which radiology clinics use the same scanning protocol for every patient undergoing prostate MRI screening.
“We’re calling this intelligent scanning,” said Hersh Chandarana, MD, professor of radiology and urology at NYU Langone and senior author of the study. “The clinical imaging protocols are designed for a population,” not for individual patients, he explained. The idea of tailoring MRI scans to each person’s needs makes sense, but such personalization has proven unfeasible—until now.
MRI is often an intermediate step in prostate cancer detection, diagnosis, and surveillance. Frontline screening usually involves a blood test for prostate-specific antigen (PSA). However, high PSA can be caused by multiple factors, and most men with elevated PSA are ultimately found not to have cancer. A follow-up MRI lowers PSA’s stratospheric false-positive rate down to about 50 percent, saving many from an unnecessary biopsy and reserving this most accurate but also most invasive method for those cases which radiologists can affirm or cannot eliminate.
Prostate MRI scans come in two variations: a short “biparametric” protocol and a longer, more detailed one known as “multiparametric.” The mutliparametric MRI comprises the biparametric scan plus dynamic contrast enhanced (DCE) MRI, which can show more clearly areas of malignant tissue but requires a contrast injection. Radiologists find DCE MRI most informative in ambiguous cases and helpful in cases with apparent malignancies. In the cases clearly shown by biparametric data to be cancer-free, however, the DCE information serves no diagnostic purpose. Yet, who gets which type of MRI does not currently depend on who needs which type.
“Generally, the decision is, everyone gets biparametric MRI or everyone gets multiparametric MRI” based on what a given imaging clinic happens to adopt as a standard, said Patricia Johnson, PhD, assistant professor of radiology at NYU Langone and scientist with the Center for Advanced Imaging Innovation and Research, who led the study. A clinic may prefer to run biparametric (also known as abbreviated) MRI for all prostate exams because the shorter protocol enables the provider to serve more patients. Or a clinic may choose to run multiparametric MRI for every prostate exam because doing so maximizes the amount of information gained.
Neither approach is optimal. In clinics that run the biparametric MRI for everyone, those who would benefit from multiparametric imaging don’t receive it (though a patient may be called back for additional scanning if the radiologist finds that more information would help better evaluate the case). Meanwhile, in clinics that screen everyone with the multiparametric protocol, the patients who don’t need DCE MRI have it done anyway.
Creating a Choice
The current practice of screening all patients with the same protocol stems from the specialization of labor in radiology. Although images appear at the MRI console during the scan, the technologist who sees them does not have the expertise to evaluate them for likelihood of cancer. The radiologist, who does have that qualification, won’t see the MRIs until the case shows up in an electronic queue at a reading terminal, after the scan.
Dr. Chandarana, Dr. Johnson, and colleagues realized that a deep learning model could introduce an informed choice during the abbreviated scan: if biparametric data clearly indicate absence of cancer, end the scan; otherwise, proceed to acquire multiparametric data.
“Our thought was, well you can do better than all or nothing,” said Dr. Johnson. “If we can make that decision at the scanner, that would be a better use of resources.”


To create a decision point, the researchers first trained an AI model to evaluate abbreviated scans for cancer. They used anonymized data from more than 26,000 biparametric prostate MRI exams conducted at NYU Langone, complete with the associated clinical evaluations and, where available, biopsy results.
The model—a “multi-task classifier”— predicts the risk of clinically significant prostate cancer according to two criteria: a five-point scale used by radiologists to score images and a ten-point scale used by pathologists to score tissue samples. Rather than pinpoint each score, the model has been calibrated to make a binary call—either low or high risk—for each scale.
The scientists then tested the model by comparing its predictions with radiology reports, biopsy results, and long-term follow-up data, and found the AI’s performance “consistent with current state-of-the-art methodologies, establishing it as a reliable tool for [prostate cancer] detection,” they write in their paper. One technical innovation here is the leveraging of biopsy-confirmed data, which are less numerous but more definitive, to make the model’s evaluation of image data more accurate.
Next, the researchers set up a prospective study at an imaging clinic, where in the course of prostate MRI exams a software system routed a subset of biparametric data from the scanner to the AI model, triggered an automatic evaluation, and generated a console notification: high risk (continue to multiparametric MRI) or low risk (end the exam).
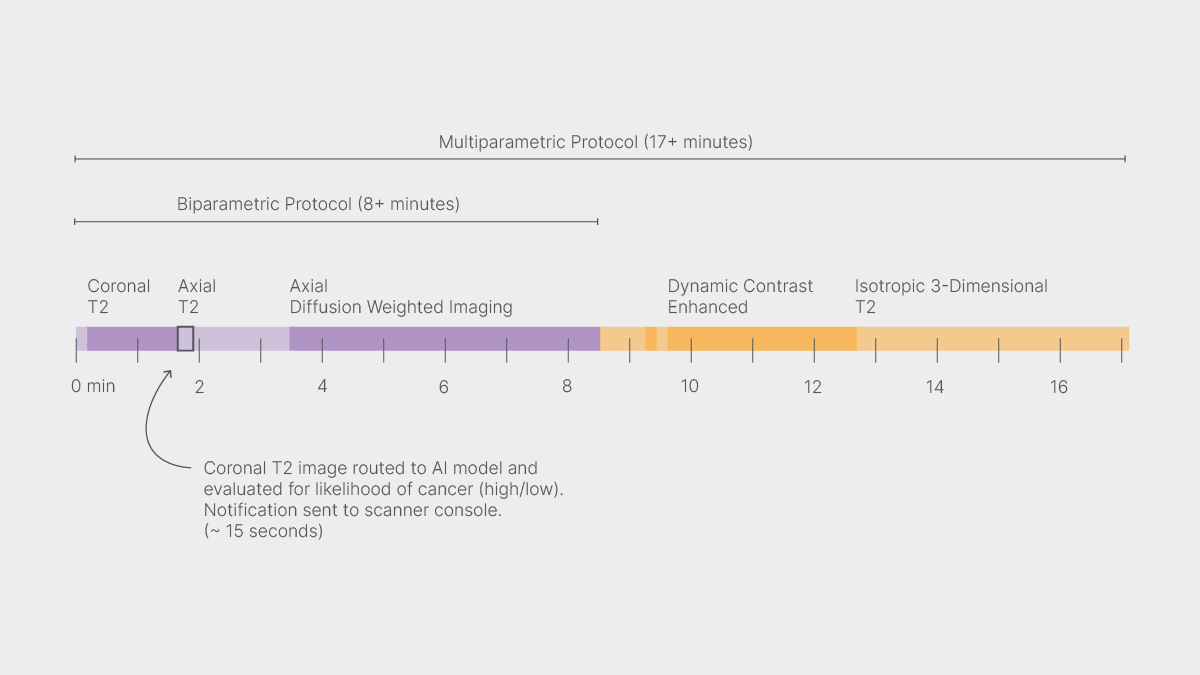
Because the AI assesses only the first image from the abbreviated protocol, the technologist receives the notification about two minutes into the scan, while the biparametric MRI is still underway.
In the study the technologists had to acknowledge the notification but did not modify the scan. (For each patient, the research team needed full mutliparametric MRI to compare the model’s assessments with those of radiologists and—in the cases referred for biopsies—pathologists.) Yet, even though the techs didn’t alter the course of the MRI exams based on AI’s input, the point is that they could have.
An Intelligent Amount of MRI
Faster scanning has been an evergreen goal of imaging scientists since MRI’s invention five decades ago. Dr. Chandarana said that initially the team was looking for ways to shorten prostate scans, maybe by one or two minutes, before taking a broader view.
For every patient newly diagnosed with prostate cancer, the wide net initially cast by PSA screening snags two to three patients who are ultimately cleared, either though imaging or a combination of other tests or an eventual biopsy. To simplify, most prostate MRI exams involve men who will eventually be found to require no treatment.
Considered in this light, the research question shifted from how to make prostate MRI faster to “why are we even doing this if so many of the patients don’t have cancer?” said Dr. Chandarana. Instead, the team focused on making the scans smarter.
And the approach does lead to shorter scans—for those who don’t need the longer ones. In the paper, the authors report that foregoing a multiparametric MRI would save approximately 9 minutes out of a 17-minute protocol. In both the retrospective and prospective studies, the deep learning model recommended abbreviated exams in about a third of the cases. But the point is not so much time as intelligence.
In their discussion, the scientists consider how different radiology clinics could tweak the model to support their particular priorities—such as serving the most patients in a given amount of time or exposing the fewest patients to unnecessary contrast injections. The model’s “operating point can be adjusted according to the specific needs and constraints of individual institutions,” the researchers write.
“A lot of people are developing [machine learning] models for predicting risk of disease, but using them for personalized medicine, scan tailoring, and imaging smarter—not many people have touched on that,” said Dr. Johnson. “This clinical use case is definitely novel and interesting.”
Related Stories
Lavanya Umapathy, postdoctoral fellow who develops representation learning models for medical imaging, talks about improving prostate-cancer screening and using artificial intelligence to approach "the person behind the images."
Patricia Johnson, who researches machine learning image reconstruction, talks about faster MRI, visual preferences, and diagnostic interchangeability.
Related Resources
A platform for integrating algorithms, AI models, and post-processing tools into clinical practice.
A platform for clinical translation of novel MRI reconstructions, raw data management, and imaging workflow analysis.





