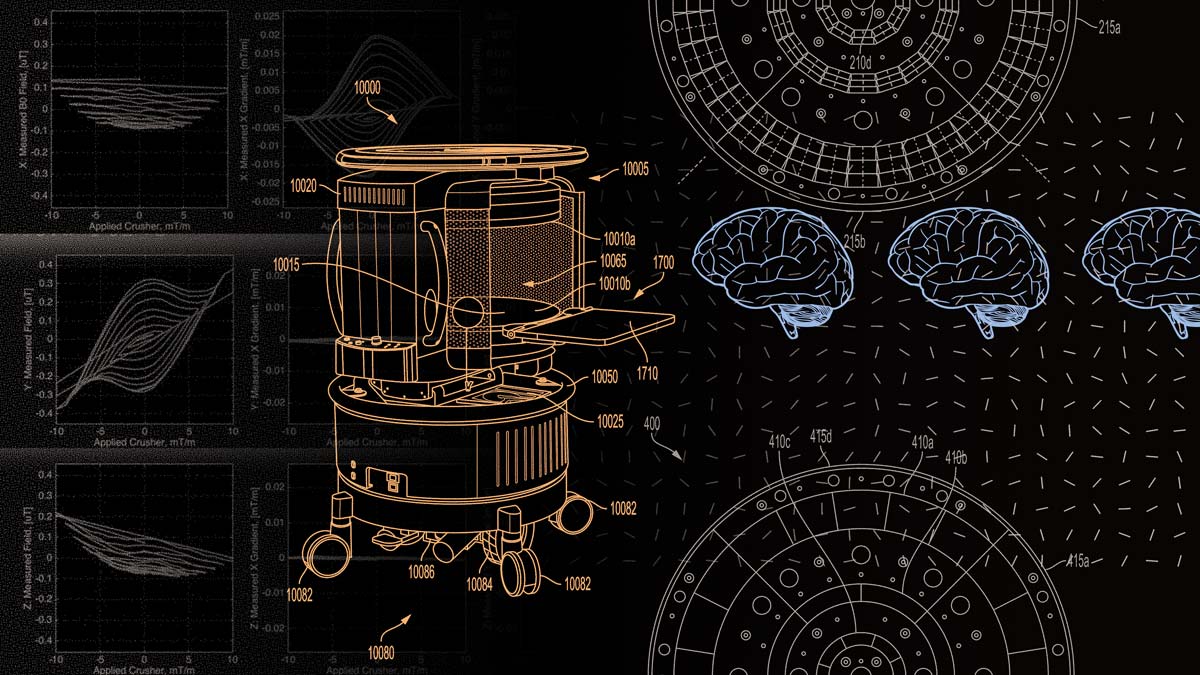
Ultra-low-field MRI scanners stand to broaden access to an imaging modality that has become a pillar of medical care. Can they also help advance large-scale research on the human brain?
To explore this question, scientists with the Center for Advanced Imaging Innovation and Research at NYU Langone Health studied the capability of the first clinically available ultra-low-field MRI scanner and recently developed deep learning tools to measure key biomarkers of brain health. In a report published recently in the journal Human Brain Mapping, the authors share their findings that ultra-low-field MRI “is a promising technology for the quantitative analysis of brain structure.” They also provide recommendations on how to use the ultra-low-field machine to maximize accuracy.
Quantitative neuroimaging biomarkers—metrics of the anatomical or physiological features that indicate aspects of the brain’s health—are essential to population-level research. Several important biomarkers center on the volume of the brain’s tissues and regions such as gray matter, white matter, and the hippocampus. Volumetric markers have been linked to a range of health phenomena, including development, aging, psychiatric disorders, neurological conditions, and neurodegenerative diseases.
It’s About Accessibility
Currently, neuroimaging research with MRI depends on the study participants’ ability to travel to the scanners and pass exclusion criteria (chief among them, no metal in the body and no claustrophobia). A typical scanner is a multi-ton stationary device located in a shielded room to protect the machine from radiofrequency interference and safeguard adjacent spaces from the MRI’s powerful magnetic field. Though the form-factor of MRI scanners hasn’t changed since their introduction half a century ago, they have steadily gotten stronger.
The standard magnetic strength of most clinical MRIs is now 1.5 tesla while medical academic centers often also operate 3-tesla scanners. Known as “high field,” these magnitudes are about ten times greater than those of the earliest clinical MRIs. The increases have been motivated by the pursuit of more detailed images and higher signal-to-noise ratio (as a rule of thumb, the higher the SNR, the more information in the acquired data).
But in the recent years a convergence of technical advances in hardware, image acquisition and reconstruction, and artificial intelligence has attracted increasing attention to the promise of ultra-low-field MRI scanners. With magnetic strengths below 0.1 tesla, these machines are about 95-percent weaker than the high-field scanners found in clinics and hospitals.
Advocates of ultra-low field MRI envision small mobile devices that—unencumbered by dependence on the kind of physical infrastructure, economic wherewithal, or technical expertise required by high-field machines—can go where conventional MRIs cannot. Though ultra-low-field scanners cannot compete with high- or even low-field counterparts in image quality, they may prove valuable in settings and applications that don’t demand—or cannot access—the full power of a traditional MRI.
The first commercially available exponent of this vision is the Hyperfine Swoop, a 0.064-tesla head-only system introduced in 2020 and until about a month ago the only ultra-low-field system with clearance from the Food and Drug Administration (in June 2025, Hyperfine unveiled a next-generation Swoop, also FDA-approved).
Swoop scanners can be wheeled around by a person or transported in the back of an ambulance. They fit through doors and plug into a wall power outlet. This type of mobility may help reduce the imbalance in today’s availability of MRI. OECD nations have an average about 20 MRI scanners per million people; the region of sub-Saharan Africa, less than one. Even in MRI-abundant countries like the U.S. the concentration of scanners in large urban centers can have the effect of leaving some patients and potential research participants on the margins.
It Matters How You Slice It
If a device like the Hyperfine Swoop can expand access to imaging care, could it also help imaging scientists reach human subjects in greater numbers or in places where traditional scanners cannot?
In their study, lead author Peter Hsu, MPhil, doctoral candidate at NYU Grossman School of Medicine, senior author Jelle Veraart, ScD, assistant professor of radiology at NYU Langone, and colleagues have addressed this question by evaluating the feasibility of acquiring volumetric brain biomarkers with the Swoop scanner.
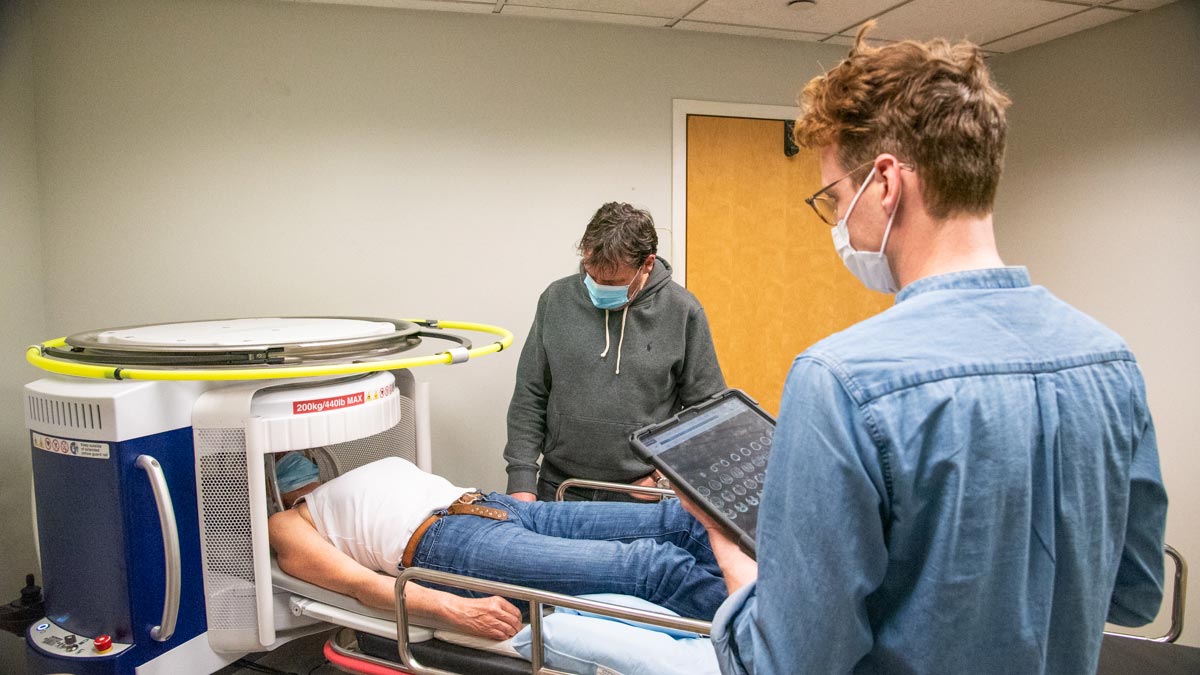
The researchers used the Swoop to scan 60 healthy adults, obtaining T1- and T2-weighted brain images—the bread and butter of MRI sequences—in axial, coronal, and sagittal planes. Approximately a third of the study participants underwent an ultra-low-field re-scan. About half of the subjects had previously taken a conventional high-field MRI. For every person, the scientists matched both the re-test and high-field images to the initial ultra-low-field scans and segmented the images from each session to estimate the volumes of grey matter, white matter, the hippocampus, lateral ventricles, and a larger measure known as total intracranial volume.
In extracting the volumetric biomarkers, the team employed MRI-specific deep learning tools introduced in 2023 called SynthSR and SynthSeg. The software boosts the resolution of brain images and provides automatic segmentation without the need for copious training data.
Then, the scientists conducted a range of numerical analyses on the biomarkers gathered from subject-matched image sets, evaluating them for within-subject similarity across magnetic field strengths and repeatability at ultra-low-field. To obtain a corresponding repeatability reference for high-field MRI, the team used a sample of test-retest images from the Human Connectome Project.
The investigators found “strong” within-subject correlation across magnetic field strengths, “excellent” within-subject agreement across field strengths, and “good to excellent” within-subject repeatability at ultra-low field—each with caveats for the particular volumetric features and types of images assessed. A clear through-line in the findings is that the ultra-low-field T2-weighted images showed higher correlation, agreement, and repeatability than their ultra-low-field T1-weighted counterparts.
The researchers have also observed that “not all orthogonal imaging directions contribute equally to volumetric accuracy” on the ultra-low-field Swoop MRI. They report which single, double, and triple acquisitions—and in what order—yield the most accurate data, with the recommended arrangement dubbed “TomoBrain.”
“The TomoBrain approach is a practical solution to increase ultra-low-field spatial resolution, given the constraints of the current imaging hardware and software,” the authors write. “We find that accurate brain volumes can be measured from [ultra-low-field MRIs] when combining orthogonal imaging directions for T2-weighted images to form a higher resolution image volume.”
Related Publication
Morphological Brain Analysis Using Ultra Low-Field MRI.
Hum Brain Mapp. 025 Jul;46(10):e70232. doi: 10.1002/hbm.70232
Related Story
How a moonshot effort to build an open-source MRI scanner from scratch in less than a week played out, and what it means for imaging research at large.